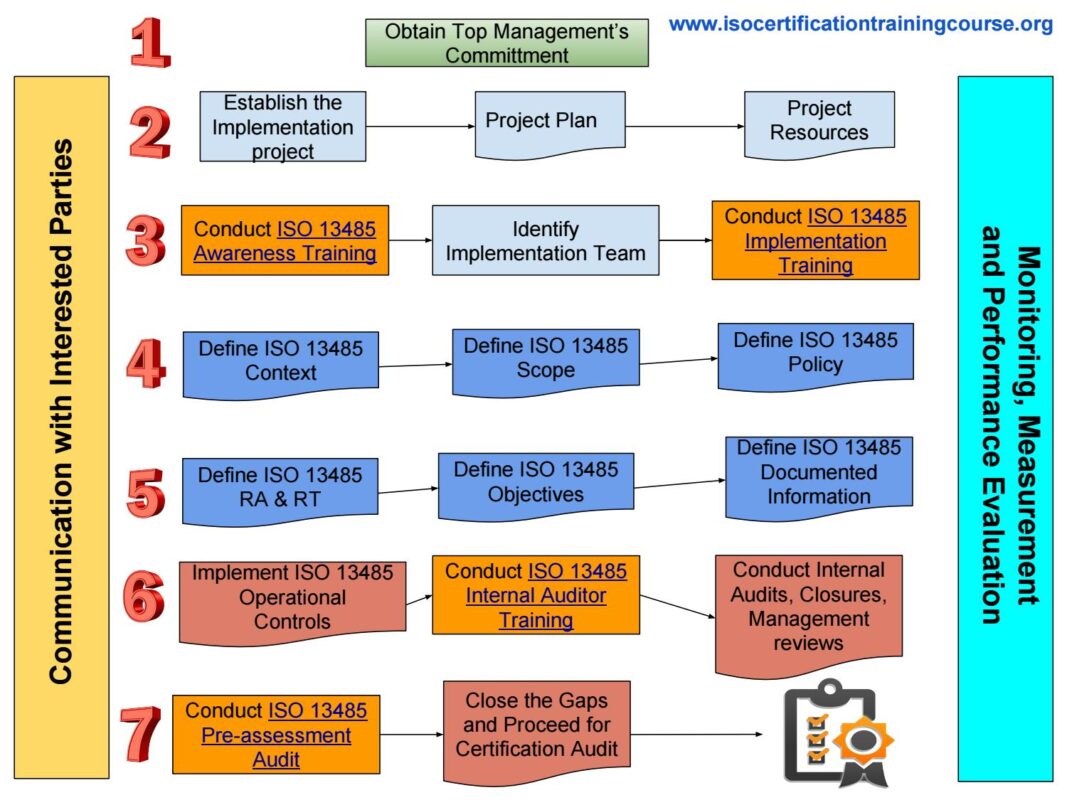
Internal auditor 13485, Uncategorized
Internal Auditor ISO 13485 2
Courtesy: Internal Auditor ISO 13485
This standard adopted by CEN as EN ISO 13485:2012 is harmonized with respect to the European Medical Devices Directive 93/42/EEC.
Mexico published on October 11, 2012, a national standard as a Norma Oficial Mexicana (NOM) to control manufacture of medical devices inside the country. NOM-241-SSA1-2012, Buenas Practicas de Fabricación para Establecimientos dedicados a la Fabricación de Dispositivos Médicos. The scope of application is mandatory in the national territory, for all establishments dedicated to the process of medical devices marketed in the country. The Cofepris is the body assigned to its control, verification and to grant the records of compliance to the companies that implement this Standard of Good Manufacturing Practices. This standard is partially in line with ISO 13485: 2003 and ISO 9001: 2008.

In 2017, The Farmacopea de los Estados Unidos Mexicanos (United Mexican States Pharmacopoeia), medical industrial sectors and Cofepris are working together for updating NOM-241 Standard, putting special attention on managing risks during manufacture and regulating by manufacturing lines some of the most important medical devices manufacturing processes. This standard will be published in August 2018, and 180 days after publication it will become mandatory for the industry.
In Spain, medical devices are named in ISO-13485 as “Sanitary Products” as Castellano-language translation of ISO-13485, but in Mexico they are known as “Medical Devices” and correspond to those used in medical practice and that meet the definition established by NOM-241 as: Medical device, to the substance, mixture of substances, material, apparatus or instrument (including the computer program necessary for its proper use or application), used alone or in combination in the diagnosis, monitoring or prevention of human or auxiliary diseases in the treatment of the same and of the disability, as well as the employees in the replacement, correction, restoration or modification of the anatomy or human physiological processes. Medical devices include products of the following categories: medical equipment, prostheses, orthotics, functional aids, diagnostic agents, supplies for dental use, surgical, healing and hygiene products. ISO 13485:2016 Certificates meets the requirement of IEC 60601-2-25 : 1993 + A1: 1999 safety of Electrocardiograms.


