HACCP Hazard analysis and critical control point, Uncategorized
HACCP Hazard analysis and critical control point 3
A second proposal by the NAS led to the development of the National Advisory Committee on Microbiological Criteria for Foods (NACMCF) in 1987. NACMCF was initially responsible for defining HACCP’s systems and guidelines for its application and were coordinated with the Codex Alimentarius Committee for Food Hygiene, that led to reports starting in 1992 and further harmonization in 1997. By 1997, the seven HACCP principles listed below became the standard.
A year earlier, the American Society for Quality offered their first certifications for HACCP Auditors. First known as Certified Quality Auditor-HACCP, they were changed to Certified HACCP Auditor (CHA) in 2004.
HACCP expanded in all realms of the food industry, going into meat, poultry, seafood, dairy, and has spread now from the farm to the fork.
Principles
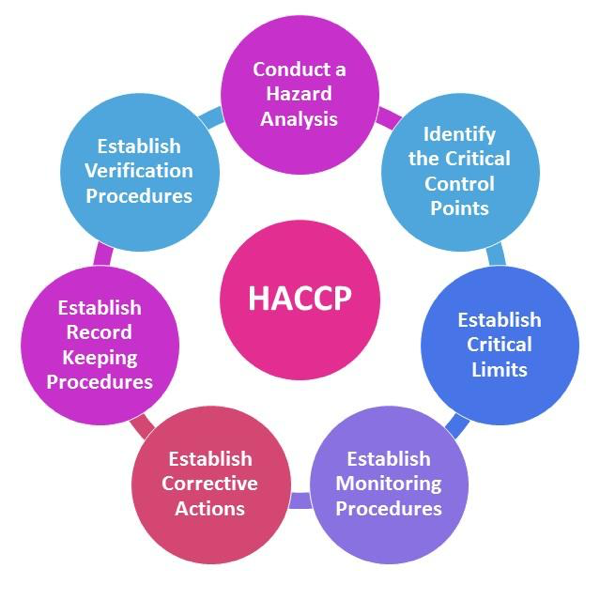
The 7 principles of HACCP
- Conduct a hazard analysisPlan to determine the food safety hazards and identify the preventive measures the plan can apply to control these hazards. A food safety hazard is any biological, chemical, or physical property that may cause a food to be unsafe for human consumption.
- Identify critical control pointsA critical control point (CCP) is a point, step, or procedure in a food manufacturing process at which control can be applied and, as a result, a food safety hazard can be prevented, eliminated, or reduced to an acceptable level.
- Establish critical limits for each critical control pointA critical limit is the maximum or minimum value to which a physical, biological, or chemical hazard must be controlled at a critical control point to prevent, eliminate, or reduce that hazard to an acceptable level.
- Establish critical control point monitoring requirementsMonitoring activities are necessary to ensure that the process is under control at each critical control point. In the United States, the FSIS requires that each monitoring procedure and its frequency be listed in the HACCP plan.
- Establish corrective actionsThese are actions to be taken when monitoring indicates a deviation from an established critical limit. The final rule requires a plant’s HACCP plan to identify the corrective actions to be taken if a critical limit is not met. Corrective actions are intended to ensure that no product is injurious to health or otherwise adulterated as a result if the deviation enters commerce.
- Establish procedures for ensuring the HACCP system is working as intendedValidation ensures that the plants do what they were designed to do; that is, they are successful in ensuring the production of a safe product. Plants will be required to validate their own HACCP plans. FSIS will not approve HACCP plans in advance, but will review them for conformance with the final rule.Verification ensures the HACCP plan is adequate, that is, working as intended. Verification procedures may include such activities as review of HACCP plans, CCP records, critical limits and microbial sampling and analysis. FSIS is requiring that the HACCP plan include verification tasks to be performed by plant personnel. Verification tasks would also be performed by FSIS inspectors. Both FSIS and industry will undertake microbial testing as one of several verification activities.Verification also includes ‘validation’ – the process of finding evidence for the accuracy of the HACCP system (e.g. scientific evidence for critical limitations).
- Establish record keeping proceduresThe HACCP regulation requires that all plants maintain certain documents, including its hazard analysis and written HACCP plan, and records documenting the monitoring of critical control points, critical limits, verification activities, and the handling of processing deviations. Implementation involves monitoring, verifying, and validating of the daily work that is compliant with regulatory requirements in all stages all the time. The differences among those three types of work are given by Saskatchewan Agriculture and Food.


