ISO 16559: 2014 Solid Biofuels, Uncategorized
ISO 16559:2014 Solid Biofuels
Courtesy: ISO 16559:2014 Solid Biofuels
Biologically produced alcohols, most commonly ethanol, and less commonly propanol and butanol, are produced by the action of microorganisms and enzymes through the fermentation of sugars or starches (easiest), or cellulose (which is more difficult).
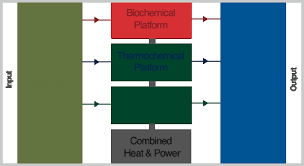
Ethanol fuel is the most common biofuel worldwide, particularly in Brazil. Alcohol fuels are produced by fermentation of sugars derived from wheat, corn, sugar beets, sugar cane, molasses and any sugar or starch from which alcoholic beverages such as whiskey, can be made (such as potato and fruit waste, etc.). The ethanol production methods used are enzyme digestion (to release sugars from stored starches), fermentation of the sugars, distillation and drying. The distillation process requires significant energy input for heat (sometimes unsustainable natural gas fossil fuel, but cellulosic biomass such as bagasse, the waste left after sugar cane is pressed to extract its juice, is the most common fuel in Brazil, while pellets, wood chips and also waste heat are more common in Europe) Waste steam fuels ethanol factory[– where waste heat from the factories also is used in the district heating grid.
Ethanol can be used in petrol engines as a replacement for gasoline; it can be mixed with gasoline to any percentage. Most existing car petrol engines can run on blends of up to 15% bioethanol with petroleum/gasoline. Ethanol has a smaller energy density than that of gasoline; this means it takes more fuel (volume and mass) to produce the same amount of work. An advantage of ethanol (CH
3CH
2OH) is that it has a higher octane rating than ethanol-free gasoline available at roadside gas stations, which allows an increase of an engine’s compression ratio for increased thermal efficiency. In high-altitude (thin air) locations, some states mandate a mix of gasoline and ethanol as a winter oxidizer to reduce atmospheric pollution emissions.
Corn-to-ethanol and other food stocks has led to the development of cellulosic ethanol.
Ethanol has roughly one-third lower energy content per unit of volume compared to gasoline. This is partly counteracted by the better efficiency when using ethanol (in a long-term test of more than 2.1 million km, the BEST project found FFV vehicles to be 1–26% more energy efficient than petrol cars, but the volumetric consumption increases by approximately 30%, so more fuel stops are required)


